
Chemistry, 19.03.2021 21:40 lilquongohard
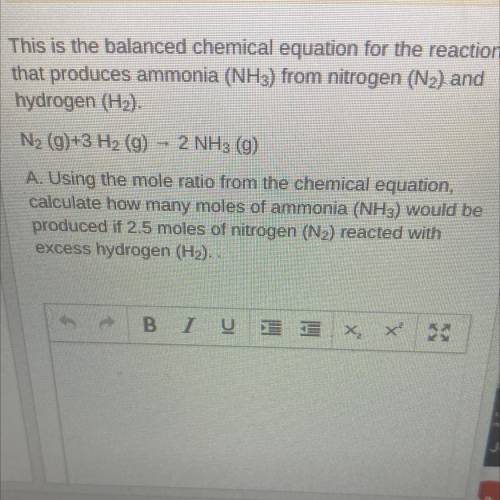
This is the balanced chemical equation for the reaction that produces ammonia (NH3) from nitrogen (N2) and hydrogen (H2).
N2 (g)+3 H2 (g) - 2 NH3 (g)
A. Using the mole ratio from the chemical equation, calculate how many moles of ammonia (NH3) would be produced if 2.5 moles of nitrogen (N2) reacted with
excess hydrogen (H2). .

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

You know the right answer?
This is the balanced chemical equation for the reaction that produces ammonia (NH3) from nitrogen (N...
Questions


Mathematics, 12.10.2019 20:30

English, 12.10.2019 20:30


Mathematics, 12.10.2019 20:30


Chemistry, 12.10.2019 20:30


Mathematics, 12.10.2019 20:30





Mathematics, 12.10.2019 20:30


History, 12.10.2019 20:30


Mathematics, 12.10.2019 20:30

Social Studies, 12.10.2019 20:30



