

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:20
Why is an elements atomic mass not listed as a whole number on the periodic table
Answers: 2

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
The density of ethanol, C2H5OH, is 0.789 g/mL. How many milliliters of ethanol are needed to produce...
Questions

English, 23.09.2019 11:10


English, 23.09.2019 11:10







History, 23.09.2019 11:10




Arts, 23.09.2019 11:10



Mathematics, 23.09.2019 11:10

Biology, 23.09.2019 11:10

Biology, 23.09.2019 11:10

Mathematics, 23.09.2019 11:10

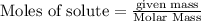
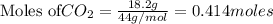
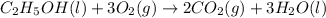
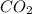 is produced by = 1 mole of
is produced by = 1 mole of 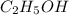
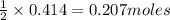 of
of 



