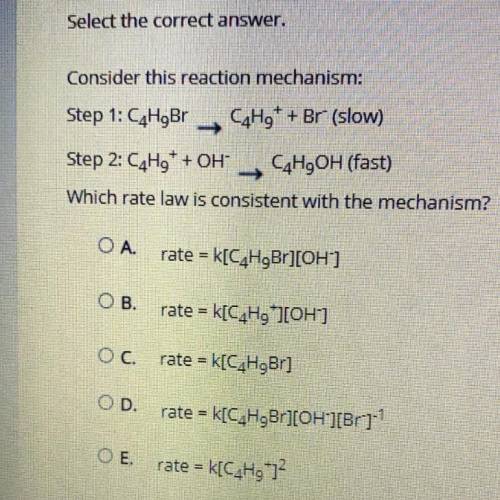
Consider this reaction mechanism:
Step 1: C4H9Br C4H9+ + Br- (slow)
Step 2: C4H9+ + OH- C4H9O...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
You know the right answer?
Questions




Mathematics, 25.10.2020 03:30


Mathematics, 25.10.2020 03:30


Mathematics, 25.10.2020 03:30

Business, 25.10.2020 03:30

Mathematics, 25.10.2020 03:30

Mathematics, 25.10.2020 03:30



Mathematics, 25.10.2020 03:30

Mathematics, 25.10.2020 03:30


Mathematics, 25.10.2020 03:30



History, 25.10.2020 03:30