
Chemistry, 21.03.2021 06:50 samiller30
A volume of 10cm3 of sulfuric acid of unknown concentration neutralized 25cm3 of sodium hydroxide solution of concentration 0.4 mol dm-3. The concentration of the acid is:
0.1 mol dm-3
0.25 mol dm-3
0.4 mol dm-3
0.5 mol dm-3

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
You know the right answer?
A volume of 10cm3 of sulfuric acid of unknown concentration neutralized 25cm3 of sodium hydroxide so...
Questions

Social Studies, 26.12.2019 10:31

History, 26.12.2019 10:31



Chemistry, 26.12.2019 10:31


Social Studies, 26.12.2019 10:31

History, 26.12.2019 10:31


Social Studies, 26.12.2019 10:31


Business, 26.12.2019 10:31


Health, 26.12.2019 10:31

English, 26.12.2019 10:31


Chemistry, 26.12.2019 10:31



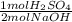 = 0.005 mol H₂SO₄
= 0.005 mol H₂SO₄

