In the equation:
2H2 + O2 --> 2H2O
What mass of Hydrogen Gas will completely react...


Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
Questions

History, 24.04.2020 04:10


Social Studies, 24.04.2020 04:10



Mathematics, 24.04.2020 04:10



Mathematics, 24.04.2020 04:11

Biology, 24.04.2020 04:11

Social Studies, 24.04.2020 04:11


History, 24.04.2020 04:11


Computers and Technology, 24.04.2020 04:11

Mathematics, 24.04.2020 04:11



Mathematics, 24.04.2020 04:11

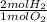 = 8 mol H₂
= 8 mol H₂

