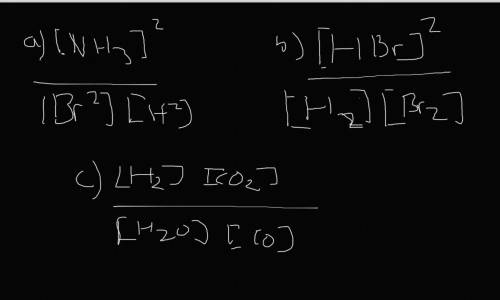Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1


Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1
You know the right answer?
Write the expressions for the equilibrium constants of the reactions below. (4 points each)
a. N2...
Questions


Mathematics, 30.12.2020 21:00

Mathematics, 30.12.2020 21:00

Business, 30.12.2020 21:00

English, 30.12.2020 21:00

Mathematics, 30.12.2020 21:00

Mathematics, 30.12.2020 21:00



Biology, 30.12.2020 21:00

Geography, 30.12.2020 21:00


Physics, 30.12.2020 21:00

Mathematics, 30.12.2020 21:00




Mathematics, 30.12.2020 21:00


Social Studies, 30.12.2020 21:00