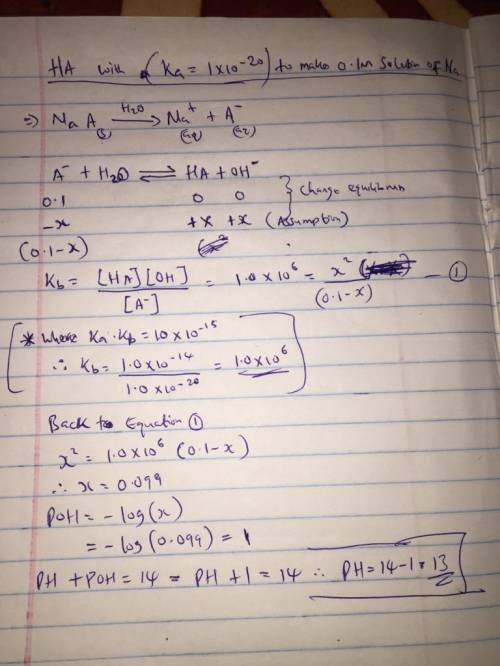Chemistry, 22.03.2021 05:40 HOTaco2181
Consider an exceptionally weak acid, HA, with a Ka = 1x10 -20 . You make a 0.1M solution of the salt Na

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3


Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
Consider an exceptionally weak acid, HA, with a Ka = 1x10 -20 . You make a 0.1M solution of the salt...
Questions

Law, 22.09.2020 04:01


Mathematics, 22.09.2020 04:01





Mathematics, 22.09.2020 04:01



Mathematics, 22.09.2020 04:01

Mathematics, 22.09.2020 04:01


English, 22.09.2020 04:01

Mathematics, 22.09.2020 04:01


Health, 22.09.2020 04:01


Mathematics, 22.09.2020 04:01

Mathematics, 22.09.2020 04:01