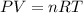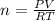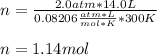Chemistry, 22.03.2021 05:50 AutumnGarringer
How many moles of ammonia gas can be formed from 14.0 L of hydrogen gas 300 K and a pressure of 2.0 atm

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

You know the right answer?
How many moles of ammonia gas can be formed from 14.0 L of hydrogen gas 300 K and a pressure of 2.0...
Questions




Mathematics, 03.02.2020 20:55

English, 03.02.2020 20:55




Chemistry, 03.02.2020 20:55




History, 03.02.2020 20:55

History, 03.02.2020 20:55




Health, 03.02.2020 20:55