
Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1
You know the right answer?
Calculate the mass of chromium produced from 50g of Cr2O3....
Questions

Mathematics, 10.12.2020 19:10

Advanced Placement (AP), 10.12.2020 19:10


Biology, 10.12.2020 19:10

Mathematics, 10.12.2020 19:10


History, 10.12.2020 19:10



Mathematics, 10.12.2020 19:10

Business, 10.12.2020 19:10




Computers and Technology, 10.12.2020 19:10

English, 10.12.2020 19:10



Physics, 10.12.2020 19:10

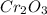
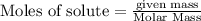
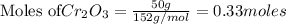
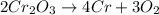
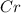
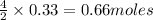 of
of 


