
Chemistry, 22.03.2021 17:10 91miketaylor
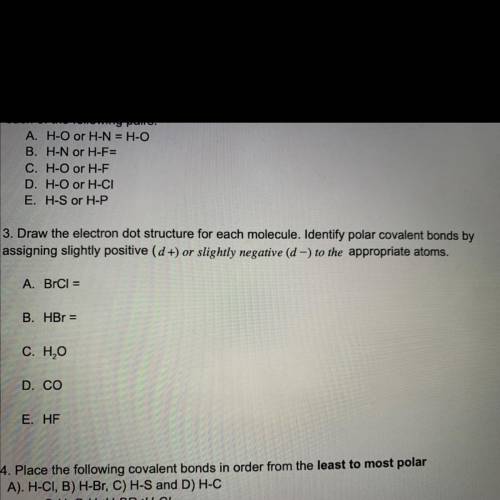
3. Draw the electron dot structure for each molecule. Identify polar covalent bonds by
assigning slightly positive (d +) or slightly negative (d -) to the appropriate atoms.
A. BrCl
B. HBr
C. H2O
D. CO
E. HF

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2

Chemistry, 23.06.2019 14:00
How many moles of oxygens atoms are present in 5.00 mol of diphosphorus of fe2(so4)3
Answers: 2
You know the right answer?
3. Draw the electron dot structure for each molecule. Identify polar covalent bonds by
assigning sl...
Questions


Business, 04.11.2020 01:00

Business, 04.11.2020 01:00

English, 04.11.2020 01:00


English, 04.11.2020 01:00

History, 04.11.2020 01:00

Mathematics, 04.11.2020 01:00


History, 04.11.2020 01:00



English, 04.11.2020 01:00

History, 04.11.2020 01:00

Arts, 04.11.2020 01:00

Advanced Placement (AP), 04.11.2020 01:00

Business, 04.11.2020 01:00

History, 04.11.2020 01:00

Chemistry, 04.11.2020 01:00

Biology, 04.11.2020 01:00



