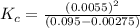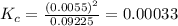Chemistry, 22.03.2021 20:10 xcrysttallx
QUESTION 6 Consider the following reaction between the diatomic and monatomic forms of iodine: I2 (g) <-> 2I (g) When 0.095 M I2 is initially placed in a previously empty container and sealed, the system slowly reaches equilibrium. When equilibrium is reached, it is found that there is an equilibrium concentration of 0.0055 M of the monatomic form of iodine. Calculate the (unitless) equilibrium constant Kc. Round your answer to two sig figs, and express it in scientific notation.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
QUESTION 6 Consider the following reaction between the diatomic and monatomic forms of iodine: I2 (g...
Questions

Mathematics, 13.07.2020 23:01

Computers and Technology, 13.07.2020 23:01




Mathematics, 13.07.2020 23:01

Mathematics, 13.07.2020 23:01

Computers and Technology, 13.07.2020 23:01




Mathematics, 13.07.2020 23:01

Mathematics, 13.07.2020 23:01


Mathematics, 13.07.2020 23:01

History, 13.07.2020 23:01

History, 13.07.2020 23:01



Mathematics, 13.07.2020 23:01

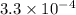
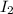 = 0.095 M
= 0.095 M 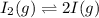
![K_c=\frac{[l]^2}{[I_2]}](/tpl/images/1211/7332/312cd.png)