
Ammonium perchlorate NH4ClO4 is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen N2 gas, chlorine Cl2 gas, oxygen O2 gas and water vapor, releasing a great deal of energy. Calculate the moles of oxygen produced by the reaction of 0.055mol of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

You know the right answer?
Ammonium perchlorate NH4ClO4 is a powerful solid rocket fuel, used in the Space Shuttle boosters. It...
Questions


Advanced Placement (AP), 17.07.2021 19:00

Mathematics, 17.07.2021 19:00

Social Studies, 17.07.2021 19:00


English, 17.07.2021 19:00


Social Studies, 17.07.2021 19:00

Advanced Placement (AP), 17.07.2021 19:00

English, 17.07.2021 19:00

English, 17.07.2021 19:00

Mathematics, 17.07.2021 19:00



Mathematics, 17.07.2021 19:10


Mathematics, 17.07.2021 19:10



English, 17.07.2021 19:10

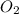 are produced by the reaction of 0.055 mol of ammonium perchlorate.
are produced by the reaction of 0.055 mol of ammonium perchlorate.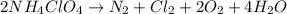
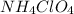 produce = 2 moles of
produce = 2 moles of 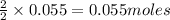 of
of 

