Chemistry, 22.03.2021 22:30 jayden6467
molecular formula citric acid2.A 10.0 mL sample of pineapple juice was titrated with 0.100 M sodium hydroxide solution. The average volume of NaOH required to reach the endpoint was 12.8 mL. a. Calculate the number of moles of sodium hydroxide required to reach the endpoint. Show your work in equation editor. Remember to use units and report your answer to the proper number of significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
molecular formula citric acid2.A 10.0 mL sample of pineapple juice was titrated with 0.100 M sodium...
Questions

Biology, 09.03.2020 19:59


Physics, 09.03.2020 19:59











Physics, 09.03.2020 20:01





Geography, 09.03.2020 20:02

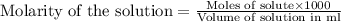
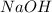 solution = 0.100 M
solution = 0.100 M