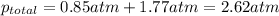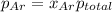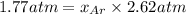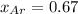Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1


Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

You know the right answer?
The partial pressure of fluorine gas in a 2.00 L container is 0.85 atm while the partial pressure in...
Questions

Computers and Technology, 30.11.2020 01:00

Mathematics, 30.11.2020 01:00



Spanish, 30.11.2020 01:00


History, 30.11.2020 01:00

Mathematics, 30.11.2020 01:00


Mathematics, 30.11.2020 01:00





Mathematics, 30.11.2020 01:00

Mathematics, 30.11.2020 01:00

Mathematics, 30.11.2020 01:00


Social Studies, 30.11.2020 01:00

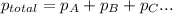
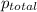 =total pressure of gases = ?
=total pressure of gases = ?
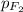 = partial pressure of fluorine = 0.85 atm
= partial pressure of fluorine = 0.85 atm
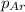 = partial pressure of Argon = 1350 torr = 1.77 atm ( 760 torr = 1atm)
= partial pressure of Argon = 1350 torr = 1.77 atm ( 760 torr = 1atm)