Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
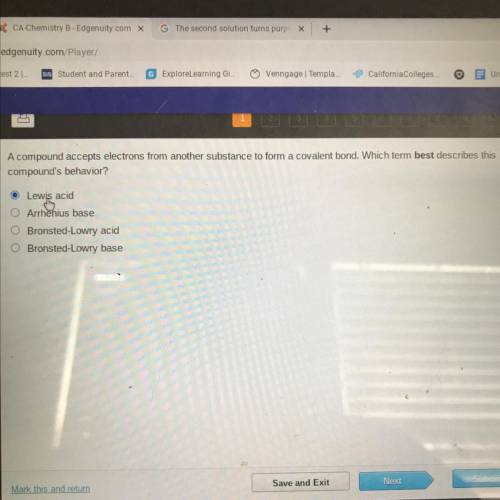
A compound accepts electrons from another substance to form a covalent bond. Which term best describ...
Questions

Social Studies, 01.07.2019 09:20

History, 01.07.2019 09:20

Spanish, 01.07.2019 09:20

History, 01.07.2019 09:20

Biology, 01.07.2019 09:20

Chemistry, 01.07.2019 09:20

Chemistry, 01.07.2019 09:20



Chemistry, 01.07.2019 09:20


Mathematics, 01.07.2019 09:20

Mathematics, 01.07.2019 09:20

Business, 01.07.2019 09:20


Spanish, 01.07.2019 09:20

Biology, 01.07.2019 09:20

Health, 01.07.2019 09:20


Mathematics, 01.07.2019 09:20