
Chemistry, 23.03.2021 01:30 rockyroad19
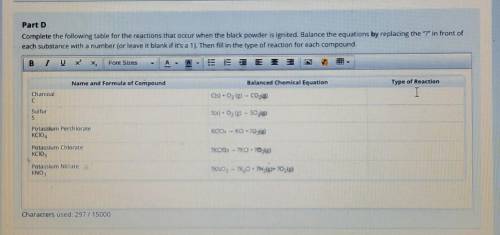
Part D Complete the following table for the reactions that occur when the black powder is ignited. Balance the equations by replacing the"? in front of each substance with a number (or leave it blank if it's a 1). Then fill in the type of reaction for each compound. B I U X X Font Sizes А E E Name and Formula of Compound Balanced Chemical Equation Type of Reaction Charcoal C с C(s) + O2 (g) - CO2(g) Sulfur S S(s) + O2 (g) - SO2(g) Potassium Perchlorate KCIOA KCIO4 - KCl + 2O2(g) Potassium Chlorate KCIO3 ?KCIO3 - ?KCI + 2O2(g) Potassium Nitrate KNO3 ?KNO3 - ?K20 +2N2(g)+ 2O2(g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
Part D Complete the following table for the reactions that occur when the black powder is ignited. B...
Questions




English, 08.06.2021 04:40


Chemistry, 08.06.2021 04:40

English, 08.06.2021 04:40

English, 08.06.2021 04:40



Mathematics, 08.06.2021 04:40


History, 08.06.2021 04:40

Biology, 08.06.2021 04:40

Business, 08.06.2021 04:40


Mathematics, 08.06.2021 04:40

Mathematics, 08.06.2021 04:40


Mathematics, 08.06.2021 04:40



