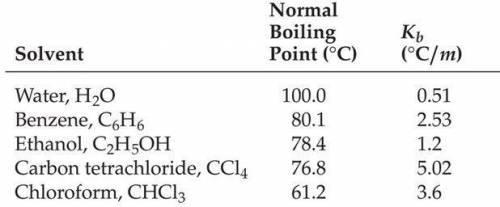
What is the freezing point in oC of a 2.20 molal solution of lithium bromide in water?
...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1

Chemistry, 23.06.2019 07:00
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1
You know the right answer?
Questions






Business, 03.08.2019 21:30





History, 03.08.2019 21:30

History, 03.08.2019 21:30


Health, 03.08.2019 21:30




Mathematics, 03.08.2019 21:30

History, 03.08.2019 21:30

Mathematics, 03.08.2019 21:30