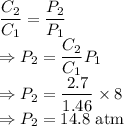Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1
You know the right answer?
A gas has a solubility of 1.46 g L at 8.00 atm of pressure. What is the pressure of a sample of the...
Questions


Mathematics, 06.04.2021 08:30

English, 06.04.2021 08:30

English, 06.04.2021 08:30


Mathematics, 06.04.2021 08:30



English, 06.04.2021 08:30





Spanish, 06.04.2021 08:30






Mathematics, 06.04.2021 08:30

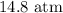
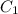 = Initial concentration = 1.46 g/L
= Initial concentration = 1.46 g/L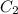 = Final concentration = 2.7 g/L
= Final concentration = 2.7 g/L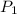 = Initial pressure = 8 atm
= Initial pressure = 8 atm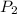 = Final pressure
= Final pressure