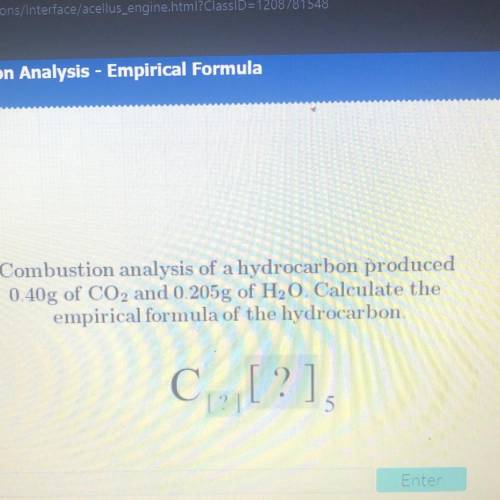
Combustion analysis of a hydrocarbon produced
0.40g of CO2 and 0.205g of H20. Calculate the
e...

Chemistry, 24.03.2021 01:30 anaclaramigli
Combustion analysis of a hydrocarbon produced
0.40g of CO2 and 0.205g of H20. Calculate the
empirical formula of the hydrocarbon.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1


Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
Questions


Biology, 10.03.2021 02:20


English, 10.03.2021 02:20





Mathematics, 10.03.2021 02:20

Chemistry, 10.03.2021 02:20

Mathematics, 10.03.2021 02:20



Computers and Technology, 10.03.2021 02:20

Chemistry, 10.03.2021 02:20

Social Studies, 10.03.2021 02:20


Chemistry, 10.03.2021 02:20

Mathematics, 10.03.2021 02:20



