
Chemistry, 24.03.2021 06:10 wittlemarie
Equimolar amounts of Cl2(g) and CO(g) are injected into an evacuated, rigid container, where they react according to the equation below.
Cl2(g) + CO --> COCl2(g)
(a)If 7.0 g of CO(g) is consumed in the reaction with excess Cl2(g), how many moles of COCl2(g) are produced?
(b) Which element is oxidized in this reaction? Justify your answer in terms of oxidation numbers.
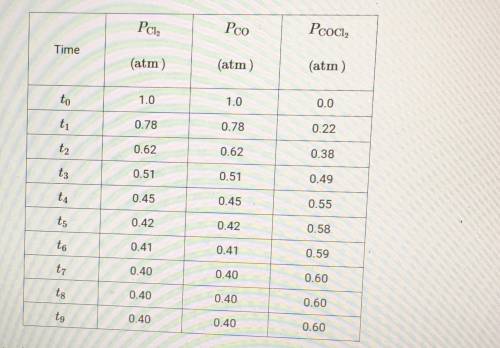
(c) At time t8, is the rate of the forward reaction greater than, less than, or equal to the rate of the reverse reaction? Justify your choice.
(d) At equilibrium, the container holds the most molecules of which gas, Cl2 or COCl2? Explain your answer.
(e) A student hypothesizes that if the temperature of the container is decreased after time t9, the mole fraction of Cl2 in the container will increase. Do you agree or disagree with student's hypothesis? Justify your answer.
(f) Using the data in the table, determine the value of the equilibrium constant, Kp, for the reaction represented by the equation below. Cl2 + C0 <--> COCl2
(g) Using the value determined in part F determine the value of Kp' for the equation shown below. 3Cl2 + 3CO <--> 3COCl2 Kp= ?
(h) Which gas, Cl2 or COCl2, will deviate most from the ideal gas law at low temperature? Justify your choice.
Thanks!!

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
Equimolar amounts of Cl2(g) and CO(g) are injected into an evacuated, rigid container, where they re...
Questions

Mathematics, 13.07.2020 17:01

Mathematics, 13.07.2020 17:01


Spanish, 13.07.2020 17:01




English, 13.07.2020 17:01


Mathematics, 13.07.2020 17:01




Spanish, 13.07.2020 17:01

Mathematics, 13.07.2020 17:01







