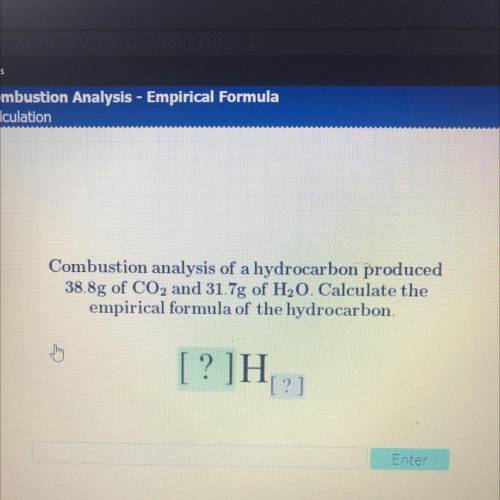
Combustion analysis of a hydrocarbon produced
38.8g of CO2 and 31.7g of H20. Calculate the
em...

Chemistry, 24.03.2021 18:30 caudhdi11721
Combustion analysis of a hydrocarbon produced
38.8g of CO2 and 31.7g of H20. Calculate the
empirical formula of the hydrocarbon.
[?]H
[?]

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1


Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1
You know the right answer?
Questions

Social Studies, 21.09.2020 05:01


Mathematics, 21.09.2020 05:01




Mathematics, 21.09.2020 05:01




English, 21.09.2020 05:01



Mathematics, 21.09.2020 05:01

Biology, 21.09.2020 05:01


Mathematics, 21.09.2020 05:01


History, 21.09.2020 05:01

Mathematics, 21.09.2020 05:01



