
Chemistry, 24.03.2021 19:00 kerstynsharp08
How many grams of aluminum oxide would form if 12.5 moles of aluminum burned 4 Al + 3 02 - 2 A1,0,?
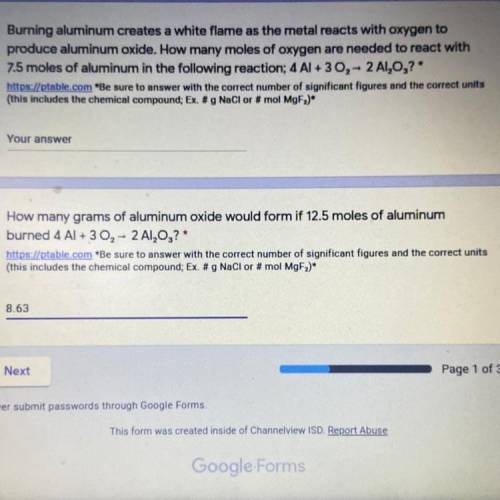

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2


Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1
You know the right answer?
How many grams of aluminum oxide would form if 12.5 moles of aluminum
burned 4 Al + 3 02 - 2 A1,0,?...
Questions


Chemistry, 04.10.2020 18:01

Geography, 04.10.2020 18:01

Mathematics, 04.10.2020 18:01

Chemistry, 04.10.2020 18:01


History, 04.10.2020 18:01

Mathematics, 04.10.2020 18:01


Biology, 04.10.2020 18:01



Mathematics, 04.10.2020 18:01

Mathematics, 04.10.2020 18:01

Mathematics, 04.10.2020 18:01

Physics, 04.10.2020 18:01


History, 04.10.2020 18:01

English, 04.10.2020 18:01

Mathematics, 04.10.2020 18:01



