
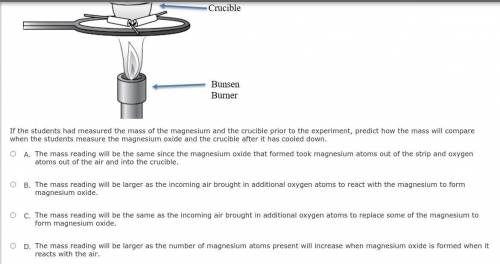
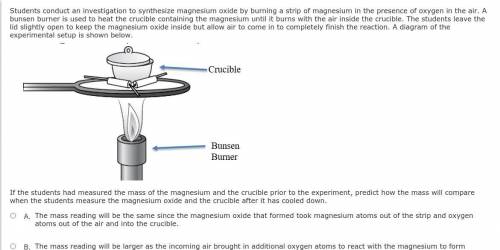
Students conduct an investigation to synthesize magnesium oxide by burning a strip of magnesium in the presence of oxygen in the air. A bunsen burner is used to heat the crucible containing the magnesium until it burns with the air inside the crucible. The students leave the lid slightly open to keep the magnesium oxide inside but allow air to come in to completely finish the reaction. A diagram of the experimental setup is shown below.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
Students conduct an investigation to synthesize magnesium oxide by burning a strip of magnesium in t...
Questions


English, 04.12.2020 21:30

Arts, 04.12.2020 21:30




Physics, 04.12.2020 21:30



Chemistry, 04.12.2020 21:30


English, 04.12.2020 21:30

Mathematics, 04.12.2020 21:30





Health, 04.12.2020 21:30

History, 04.12.2020 21:30

Biology, 04.12.2020 21:30



