
Chemistry, 24.03.2021 20:30 carelee9449
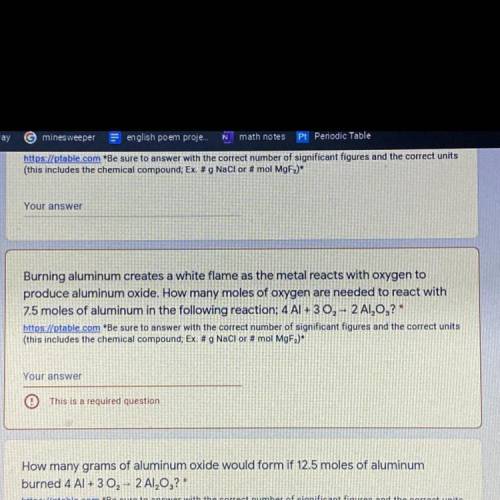
Burning aluminum creates a white flame as the metal reacts with oxygen to
produce aluminum oxide. How many moles of oxygen are needed to react with
7.5 moles of aluminum in the following reaction; 4 AI + 3 02 - 2 A1,0,?*

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

You know the right answer?
Burning aluminum creates a white flame as the metal reacts with oxygen to
produce aluminum oxide. H...
Questions


Mathematics, 01.04.2021 03:10

Mathematics, 01.04.2021 03:10

Mathematics, 01.04.2021 03:10


Mathematics, 01.04.2021 03:10


Mathematics, 01.04.2021 03:10

Computers and Technology, 01.04.2021 03:10




Mathematics, 01.04.2021 03:10


Arts, 01.04.2021 03:10

Mathematics, 01.04.2021 03:10

Mathematics, 01.04.2021 03:10

Law, 01.04.2021 03:10


Mathematics, 01.04.2021 03:10



