
Chemistry, 24.03.2021 22:30 nyceastcoast
How many moles of gas does it take to occupy 120 liters at a pressure of 2.3 atmospheres and a temperature of 240 K?*
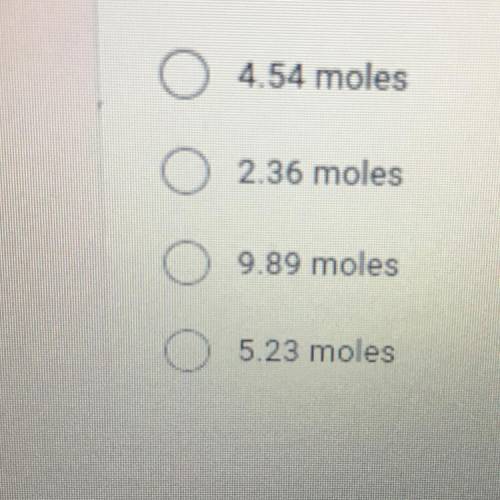

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 05:00
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
How many moles of gas does it take to occupy 120 liters at a pressure of
2.3 atmospheres and a temp...
Questions

English, 08.12.2020 14:00


Computers and Technology, 08.12.2020 14:00

Mathematics, 08.12.2020 14:00

Mathematics, 08.12.2020 14:00

Computers and Technology, 08.12.2020 14:00





Chemistry, 08.12.2020 14:00

Computers and Technology, 08.12.2020 14:00



Mathematics, 08.12.2020 14:00


English, 08.12.2020 14:00


Mathematics, 08.12.2020 14:00

Physics, 08.12.2020 14:00



