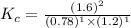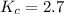Hydrogen and chlorine react to form hydrogen chloride, like this:
H2(g) + Cl,(g) → 2 HCl(g)
...

Chemistry, 24.03.2021 22:30 Kelseygrace8372
Hydrogen and chlorine react to form hydrogen chloride, like this:
H2(g) + Cl,(g) → 2 HCl(g)
Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen, chlorine, and hydrogen chloride has the following composition:
compound pressure at equilibrium
H2 0.78
Cl2 1.2M
HCl 1.6M
Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 23.06.2019 12:30
Choose one literary selection from this semester in which you think the setting has a great impact on the work. in a full paragraph name the work, describe the setting, and explain why it is so important to the overall story or poem.
Answers: 1


Chemistry, 23.06.2019 19:00
Covalent bonds have a higher melting points than ionic bonds. true or false
Answers: 1
You know the right answer?
Questions

Physics, 22.10.2019 12:50

Mathematics, 22.10.2019 12:50



Mathematics, 22.10.2019 12:50

Biology, 22.10.2019 12:50

Biology, 22.10.2019 12:50

Mathematics, 22.10.2019 12:50




Physics, 22.10.2019 12:50

Physics, 22.10.2019 13:00


Geography, 22.10.2019 13:00

Social Studies, 22.10.2019 13:00



Mathematics, 22.10.2019 13:00

English, 22.10.2019 13:00

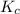
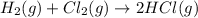
![K_c=\frac{[HCl]^2}{[H_2]^1[I_2]^1}](/tpl/images/1218/2768/5d189.png)