PLEASE HELPP!
The proposed mechanism for a reaction is:
Step 1: A + B X (fast)
Step 2:...

Chemistry, 24.03.2021 22:40 nnaaatt1845
PLEASE HELPP!
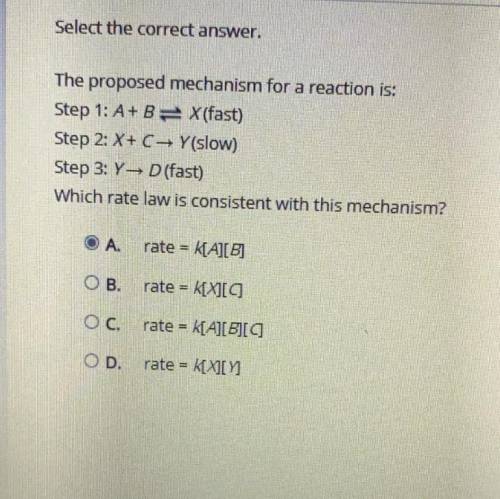
The proposed mechanism for a reaction is:
Step 1: A + B X (fast)
Step 2: X + C → Y (slow)
Step 3: Y → D (fast)
Which rate law is consistent with this mechanism?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
You know the right answer?
Questions


World Languages, 13.07.2019 00:10

Chemistry, 13.07.2019 00:10

Chemistry, 13.07.2019 00:10

Mathematics, 13.07.2019 00:10

Mathematics, 13.07.2019 00:10


Mathematics, 13.07.2019 00:10


Chemistry, 13.07.2019 00:10

Mathematics, 13.07.2019 00:10

Chemistry, 13.07.2019 00:10

Mathematics, 13.07.2019 00:10

Mathematics, 13.07.2019 00:10



Mathematics, 13.07.2019 00:10

Mathematics, 13.07.2019 00:10

Geography, 13.07.2019 00:10

Social Studies, 13.07.2019 00:10



