Please help
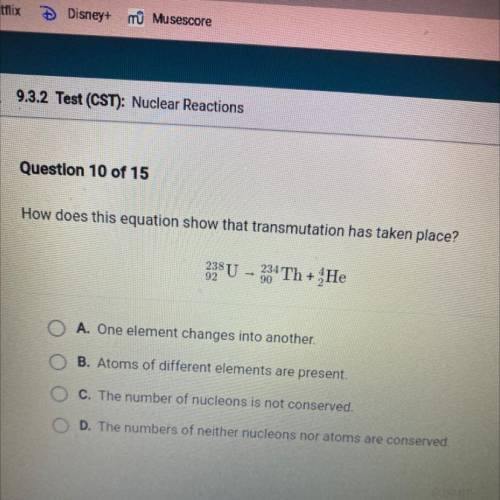
How does this equation show that transmutation has taken place?
238 U
304 T...

Chemistry, 25.03.2021 07:40 maevemboucher78
Please help
How does this equation show that transmutation has taken place?
238 U
304 Th + He
A. One element changes into another.
B. Atoms of different elements are present.
C. The number of nucleons is not conserved.
D. The numbers of neither nucleons nor atoms are conserved.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1


Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
Questions

Mathematics, 02.11.2020 21:40


Mathematics, 02.11.2020 21:40

Mathematics, 02.11.2020 21:40



Mathematics, 02.11.2020 21:40




Chemistry, 02.11.2020 21:40


Biology, 02.11.2020 21:40


SAT, 02.11.2020 21:40

Mathematics, 02.11.2020 21:40

Mathematics, 02.11.2020 21:40

English, 02.11.2020 21:40

Mathematics, 02.11.2020 21:40



