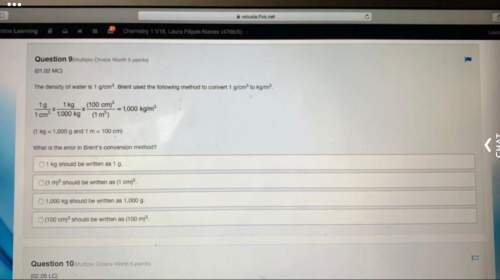
"A percentage yield of 137% from a reaction is a perfectly
reasonable result."
Is the above s...

Chemistry, 25.03.2021 15:20 smallblueberry
"A percentage yield of 137% from a reaction is a perfectly
reasonable result."
Is the above statement true or false?
O True
O False

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3
You know the right answer?
Questions








Biology, 20.08.2021 17:00

Mathematics, 20.08.2021 17:00


Business, 20.08.2021 17:00

Mathematics, 20.08.2021 17:00





Geography, 20.08.2021 17:00