
Chemistry, 25.03.2021 16:30 kirkhester1
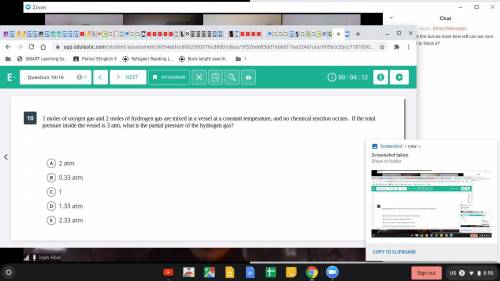
1 mole of oxygen gas and 2 moles of hydrogen are mixed in a vessel at a constant temperature, and no chemical reaction occurs. If the total pressure inside the vessel is 3 atm, what is the partial pressure of the hydrogen gas?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1

Chemistry, 23.06.2019 05:50
Which of the following is not a characteristic of s waves?
Answers: 1
You know the right answer?
1 mole of oxygen gas and 2 moles of hydrogen are mixed in a vessel at a constant temperature, and no...
Questions

History, 17.04.2020 22:54

Chemistry, 17.04.2020 22:54

Physics, 17.04.2020 22:54

Mathematics, 17.04.2020 22:54




Biology, 17.04.2020 22:54




Social Studies, 17.04.2020 22:55


Mathematics, 17.04.2020 22:55

English, 17.04.2020 22:55

Mathematics, 17.04.2020 22:55




Health, 17.04.2020 22:55



