
Chemistry, 25.03.2021 18:20 phebusadrian01
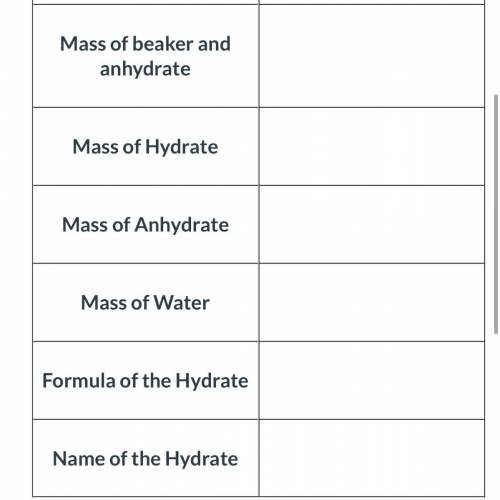
An empty beaker was found to have a mass of 50.49 grams. A hydrate of sodium carbonate was added to the beaker. When the beaker and hydrate was weighed again, the new mass was 62.29 grams. The beaker and the hydrated compound were heated and cooled several times to remove all of the water. The beaker and the anhydrate were then weighed and its new mass was determined to be 59.29 grams.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1
You know the right answer?
An empty beaker was found to have a mass of 50.49 grams. A hydrate of sodium carbonate was added to...
Questions





Mathematics, 19.08.2019 05:50




Mathematics, 19.08.2019 05:50





English, 19.08.2019 05:50



Mathematics, 19.08.2019 05:50

Physics, 19.08.2019 05:50


History, 19.08.2019 05:50



