
Chemistry, 25.03.2021 18:30 Britny2386
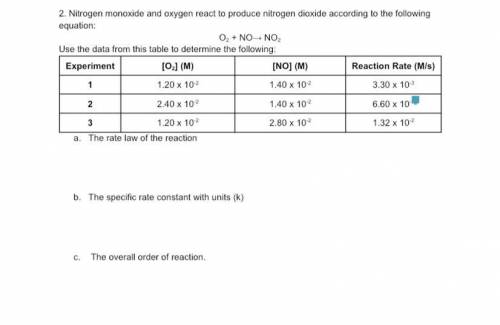
Nitrogen monoxide and oxygen react to produce nitrogen dioxide according to the following equation:
The rate law of the reaction
The specific rate constant with units (k)
The overall order of reaction.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2


Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2
You know the right answer?
Nitrogen monoxide and oxygen react to produce nitrogen dioxide according to the following equation:...
Questions

Mathematics, 18.10.2020 15:01

English, 18.10.2020 15:01

English, 18.10.2020 15:01

History, 18.10.2020 15:01

Social Studies, 18.10.2020 15:01


Chemistry, 18.10.2020 15:01

Geography, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01

History, 18.10.2020 15:01


English, 18.10.2020 15:01

History, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01

Biology, 18.10.2020 15:01

History, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01

Biology, 18.10.2020 15:01



