
Chemistry, 25.03.2021 22:30 SkinnestXOXO
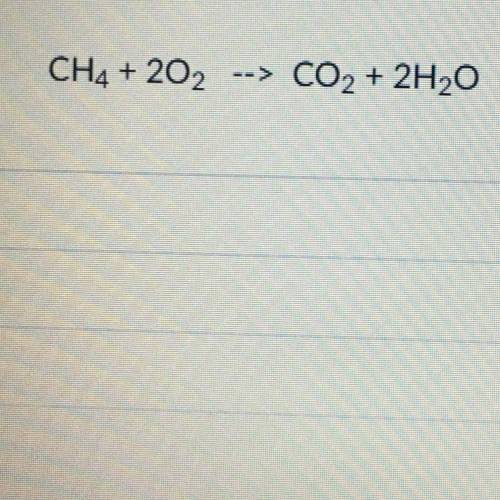
The limiting reagent for the reaction seen below is the Oxygen. If you
started off with 13.6g of O2 what is your theoretical yield for Carbon
Dioxide in grams?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
The limiting reagent for the reaction seen below is the Oxygen. If you
started off with 13.6g of O2...
Questions


History, 04.07.2020 03:01

Computers and Technology, 04.07.2020 03:01










Mathematics, 04.07.2020 03:01


Mathematics, 04.07.2020 03:01







