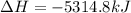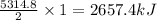Chemistry, 26.03.2021 01:00 turboslayer
Butane C4H10 (g),(Hf = –125.7), combusts in the presence of oxygen to form CO2 (g) (Delta. Hf = –393.5 kJ/mol), and H2O(g) (Delta. Hf = –241.82) in the reaction: 2 upper C subscript 4 upper H subscript 10 (g) plus 13 upper O subscript 2 (g) right arrow 8 upper C upper O subscript 2 plus 10 upper H subscript 2 upper O (g). What is the enthalpy of combustion, per mole, of butane? Use Delta H r x n equals the sum of delta H f of all the products minus the sum of delta H f of all the reactants.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 06:30
What happens to the glucose molecule during the process of cellular respiration? (5 points) select one: a. it gets broken down. b. it forms oxygen. c. it builds muscles. d. it uses up energy.
Answers: 3

Chemistry, 23.06.2019 11:30
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1
You know the right answer?
Butane C4H10 (g),(Hf = –125.7), combusts in the presence of oxygen to form CO2 (g) (Delta. Hf = –393...
Questions






Mathematics, 31.05.2020 03:01





Biology, 31.05.2020 03:01

Mathematics, 31.05.2020 03:01





Mathematics, 31.05.2020 03:01



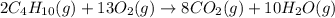
![\Delta H=[n\times H_f_{products}]-[n\times H_f_{reactants}]](/tpl/images/1221/8923/4f68b.png)
![\Delta H=[8\times H_f_{CO_2}+10\times H_f_{H_2O}]-[2\times H_f_{C_4H_{10}+13\times H_f_{O_2}}]](/tpl/images/1221/8923/e94db.png)
![\Delta H=[(8\times -393.5)+(10\times -241.82)]-[(2\times -125.7)+(13\times 0)]](/tpl/images/1221/8923/8343f.png)