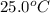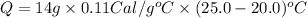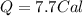Chemistry, 16.10.2019 15:30 oofoofoof1
Suppose a 14-gram sample of iron is heated from 20.0°c to 25.0°c. the specific heat of iron is 0.11 cal/g°c. how much heat energy was absorbed by the iron? 38.5 cal 7.7 cal 636 cal 69.3 cal

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Anurse practitioner prepares an injection of promethazine, an antihistamine used to treat allergic rhinitis. if the stock bottle is labeled 25 mg/ml and the order is a dose of 11.0 mg , how many milliliters will the nurse draw up in the syringe?
Answers: 3


Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1
You know the right answer?
Suppose a 14-gram sample of iron is heated from 20.0°c to 25.0°c. the specific heat of iron is 0.11...
Questions




English, 18.09.2019 07:10

Mathematics, 18.09.2019 07:10

Biology, 18.09.2019 07:10

History, 18.09.2019 07:10






History, 18.09.2019 07:10

Mathematics, 18.09.2019 07:10


Mathematics, 18.09.2019 07:10


Geography, 18.09.2019 07:10

English, 18.09.2019 07:10

History, 18.09.2019 07:20

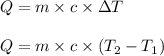
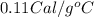
 = change in temperature
= change in temperature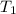 = initial temperature =
= initial temperature = 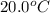
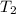 = final temperature =
= final temperature =