I
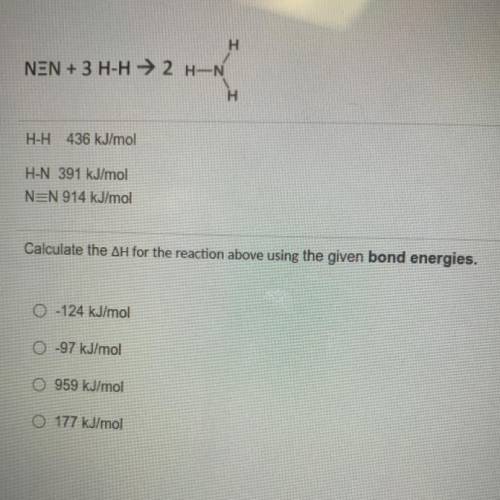
NEN + 3 H-H → 2 H-N
H
H-H 436 kJ/mol
H-N 391 kJ/mol
N=N 914 kJ/mol
...

Chemistry, 26.03.2021 17:20 madisynk78
I
NEN + 3 H-H → 2 H-N
H
H-H 436 kJ/mol
H-N 391 kJ/mol
N=N 914 kJ/mol
Calculate the AH for the reaction above using the given bond energies.
O-124 kJ/mol
0 -97 kJ/mol
O 959 kJ/mol
O 177 kJ/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
Questions

Computers and Technology, 25.11.2020 07:30

English, 25.11.2020 07:30

Social Studies, 25.11.2020 07:30


English, 25.11.2020 07:30


Mathematics, 25.11.2020 07:30

English, 25.11.2020 07:30

English, 25.11.2020 07:30

Biology, 25.11.2020 07:30


Mathematics, 25.11.2020 07:30



Social Studies, 25.11.2020 07:30

History, 25.11.2020 07:30


Arts, 25.11.2020 07:30


Chemistry, 25.11.2020 07:30



