
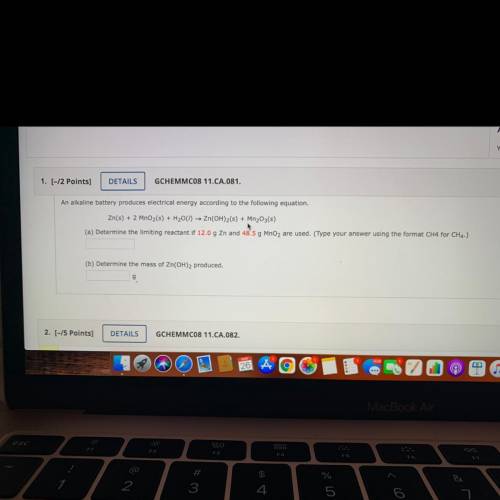
An alkaline battery produces electrical energy according to the following equation.
Zn(s) + 2 MnO2(s) + H2001) — Zn(OH)2(s) + Mn2O3(s)
(a) Determine the limiting reactant if 12.0 g Zn and 48.5 g MnO2 are used. (Type your answer using the format CH4 for CH4.)
(b) Determine the mass of Zn(OH)2 produced.

Answers: 2
Another question on Chemistry



Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1
You know the right answer?
An alkaline battery produces electrical energy according to the following equation.
Zn(s) + 2 MnO2(...
Questions

Business, 07.07.2019 15:00





Social Studies, 07.07.2019 15:00

Physics, 07.07.2019 15:00


English, 07.07.2019 15:00


English, 07.07.2019 15:00


Chemistry, 07.07.2019 15:00


History, 07.07.2019 15:00


English, 07.07.2019 15:00

Mathematics, 07.07.2019 15:00

Mathematics, 07.07.2019 15:00



