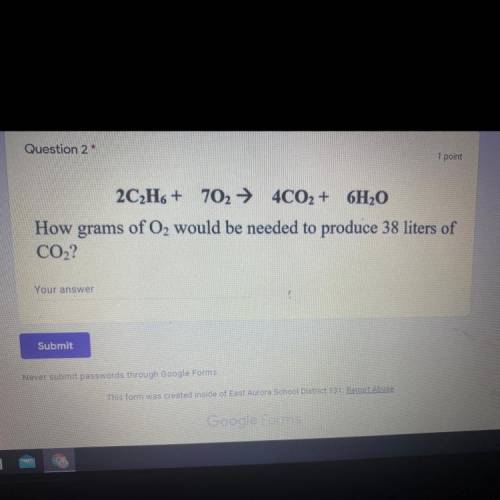
2C2H6 + 702 + 4CO2 + 6H2O
How grams of O2 would be needed to produce 38 liters of
CO2?
<...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2


Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
Questions




English, 30.09.2021 18:30

Mathematics, 30.09.2021 18:30

Mathematics, 30.09.2021 18:30

Mathematics, 30.09.2021 18:30

Computers and Technology, 30.09.2021 18:30


Mathematics, 30.09.2021 18:30





Mathematics, 30.09.2021 18:30


Social Studies, 30.09.2021 18:30

Mathematics, 30.09.2021 18:30

Mathematics, 30.09.2021 18:30