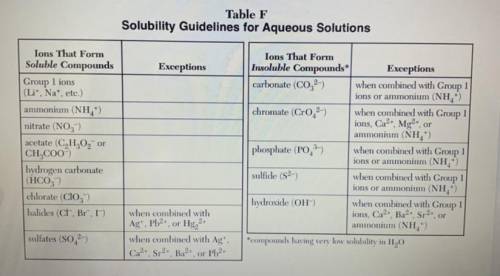
Based on Table F, which compound is least soluble in water?
1. AIPO4
2. Li2SO4
3. Ca(OH...

Chemistry, 26.03.2021 20:40 vannitling12p4w44f
Based on Table F, which compound is least soluble in water?
1. AIPO4
2. Li2SO4
3. Ca(OH)2
4. AgC₂H₂O2

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2
You know the right answer?
Questions

Mathematics, 10.07.2019 15:30

Computers and Technology, 10.07.2019 15:30


World Languages, 10.07.2019 15:30








History, 10.07.2019 15:30

Mathematics, 10.07.2019 15:30



History, 10.07.2019 15:30




Mathematics, 10.07.2019 15:30



