What is the net ionic equation for
the reaction below?
3NaOH(aq) +H3PO4 (aq) → Na3PO4(aq) + 3...

Chemistry, 26.03.2021 20:40 afolmar2006
What is the net ionic equation for
the reaction below?
3NaOH(aq) +H3PO4 (aq) → Na3PO4(aq) + 3H2O(1)
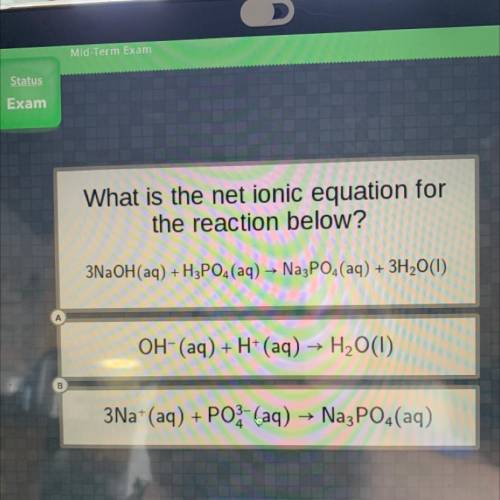

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
Questions




Mathematics, 20.02.2020 04:48





Computers and Technology, 20.02.2020 04:48

Computers and Technology, 20.02.2020 04:48


Mathematics, 20.02.2020 04:48



Mathematics, 20.02.2020 04:49



Mathematics, 20.02.2020 04:49

History, 20.02.2020 04:49



