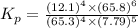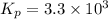Chemistry, 26.03.2021 21:10 dchannakhone84
Ammonia and oxygen react to form nitrogen monoxide and water, like this:
Also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition:
Compound pressure at equilibrium
NH_3 65.3 atm
O_2 7.79 atm
NO 12.1 atm
H_2O 65.8 atm
calculate the value of the equilibrium constant Kp for this reaction. Round your answer to 2 significant figures.
Kp=

Answers: 2
Another question on Chemistry



Chemistry, 23.06.2019 07:10
Which one of the following is an oxidation-reduction reaction? naoh + hno3 --> h2o + kno3 naoh + hno3 --> h2o + kno3 so3 + h2o --> h2so4 cacl2 + na2co3 --> caco3 + 2 nacl ch4 + 2 o2 --> co2 + 2 h2o al2(so4)3 + 6 koh --> 2 al(oh)3 + 3 k2so4
Answers: 3

Chemistry, 23.06.2019 08:30
What percentage of energy used in the u.s is produced from fossil fuels
Answers: 2
You know the right answer?
Ammonia and oxygen react to form nitrogen monoxide and water, like this:
Also, a chemist finds that...
Questions

Mathematics, 20.07.2019 12:00

Social Studies, 20.07.2019 12:00



English, 20.07.2019 12:00

Biology, 20.07.2019 12:00

History, 20.07.2019 12:00


Social Studies, 20.07.2019 12:00







Mathematics, 20.07.2019 12:00

History, 20.07.2019 12:00

Mathematics, 20.07.2019 12:00



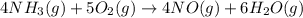
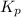 is written as:
is written as:
![K_p=\frac{[p_{NO}]^4\times [p_{H_2O}]^6}{[p_{NH_3}]^4\times [p_{O_2}]^5}](/tpl/images/1224/2313/889d8.png)