
Chemistry, 26.03.2021 22:50 barnhill4755
Which of these equations represent reactions that could be used in constructing an electrochemical cell? Check all that apply.
A. CH4 +2O2 → CO2 + 2H20
B. Cr + Cu^2+ ---> Cr^2+ + Cu
C. 2 Ag+ + Fe → 2Ag + Fe^2+
D. CI^- + Ag^+ → AgCI
E. NH3 +H^+ ---> NH4^4
just got it wrong, the answers are B and C. Just solved my own question
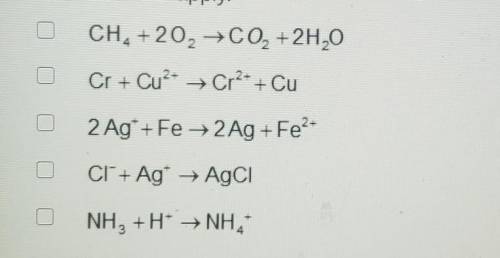

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1
You know the right answer?
Which of these equations represent reactions that could be used in constructing an electrochemical c...
Questions

Mathematics, 14.07.2020 22:01





Geography, 14.07.2020 22:01





Mathematics, 14.07.2020 22:01



Mathematics, 14.07.2020 22:01




Mathematics, 14.07.2020 22:01

History, 14.07.2020 22:01



