
Chemistry, 26.03.2021 23:20 natajaeecarr
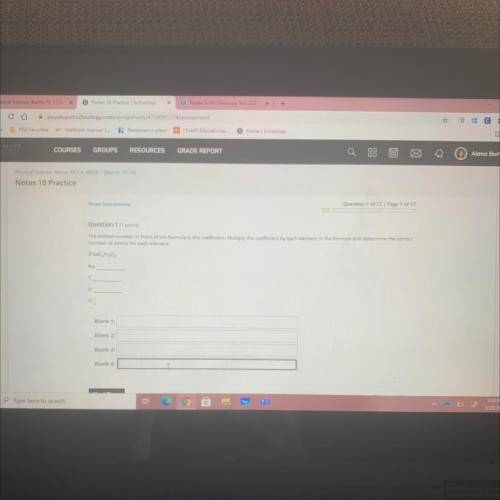
The bolded number in front of the formula is the coefficient. Multiply the coefficient by each element in the formula and determine the correct
number of atoms for each element.
3 NaC₂H₂O2
Please help I don’t understand!!

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:50
Which of these reactions are redox reactions? check all that apply.cd + hcl → cdcl2 + h2cucl2 + na2s → 2nacl + cuscaco3 → cao + co2 2zns + 3o2 → 2zno + 2so2 ch4 + 2o2 → co2 + 2h2o
Answers: 3


Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

You know the right answer?
The bolded number in front of the formula is the coefficient. Multiply the coefficient by each eleme...
Questions

Chemistry, 11.12.2020 01:00

Social Studies, 11.12.2020 01:00

English, 11.12.2020 01:00



English, 11.12.2020 01:00

Chemistry, 11.12.2020 01:00


Physics, 11.12.2020 01:00


Arts, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00

Chemistry, 11.12.2020 01:00


History, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00


Computers and Technology, 11.12.2020 01:00




