
Chemistry, 29.03.2021 16:50 lindamillscotton90
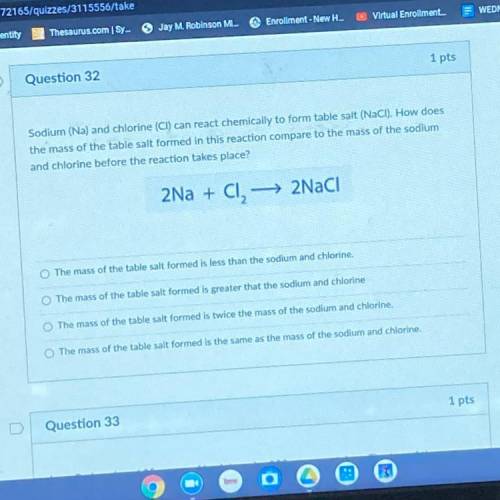
Sodium (Na) and chlorine (CI) can react chemically to form table salt (NaCl). How does the mass of the table salt formed in this reaction compare to the mass of the sodium and chlorine before the reaction takes place?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1
You know the right answer?
Sodium (Na) and chlorine (CI) can react chemically to form table salt (NaCl). How does
the mass of...
Questions





Mathematics, 23.10.2020 23:20


Chemistry, 23.10.2020 23:20

Mathematics, 23.10.2020 23:20

Biology, 23.10.2020 23:20




Mathematics, 23.10.2020 23:20

Mathematics, 23.10.2020 23:20

Mathematics, 23.10.2020 23:20

Engineering, 23.10.2020 23:20


Mathematics, 23.10.2020 23:20


Mathematics, 23.10.2020 23:20



