
Chemistry, 29.03.2021 18:10 yugbug44owwc7w
To increase the temperature of 100.0 g of H2O(s) from -50.0°C to -10.0°C, how much energy is required?
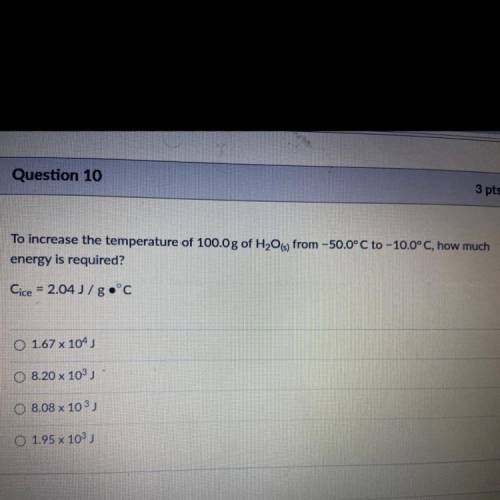

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 1

Chemistry, 23.06.2019 12:30
15) a substance used in manufacturing gasoline consists of finely divided platinum supported on an inert solid. suppose that the platinum is formed by the high temperature reaction between platinum (iv) oxide and hydrogen gas. the other product is water. a) write and balance the equation b) how many grams of hydrogen are needed to produce 1.0 g of platinum metal? c) how many moles of water are produced at the same time? how many grams? ( show work, .)
Answers: 1
You know the right answer?
To increase the temperature of 100.0 g of H2O(s) from -50.0°C to -10.0°C, how much
energy is requir...
Questions

Advanced Placement (AP), 05.12.2020 14:00

Biology, 05.12.2020 14:00

Advanced Placement (AP), 05.12.2020 14:00







History, 05.12.2020 14:00


Advanced Placement (AP), 05.12.2020 14:00


Biology, 05.12.2020 14:00

English, 05.12.2020 14:00

Computers and Technology, 05.12.2020 14:00

Mathematics, 05.12.2020 14:00

Biology, 05.12.2020 14:00


Physics, 05.12.2020 14:00




