Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
A sealed 1.0L flask is filled with 0.500 mols of I_2 and 0.500 mols of Br_2. When the container achi...
Questions

Mathematics, 25.01.2021 20:10

Chemistry, 25.01.2021 20:10


Mathematics, 25.01.2021 20:10

Mathematics, 25.01.2021 20:10

Biology, 25.01.2021 20:10


Mathematics, 25.01.2021 20:10

Mathematics, 25.01.2021 20:10


Mathematics, 25.01.2021 20:10

Mathematics, 25.01.2021 20:10

Mathematics, 25.01.2021 20:10



Business, 25.01.2021 20:10




Mathematics, 25.01.2021 20:10

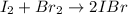
![K=\frac{[IBr]^2}{[I_2][Br_2]} =0.0110](/tpl/images/1228/6485/945be.png)
 (reaction extent) would be:
(reaction extent) would be: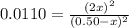
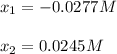
![[IBr]=2x=2*0.0249M=0.049M](/tpl/images/1228/6485/0c444.png)