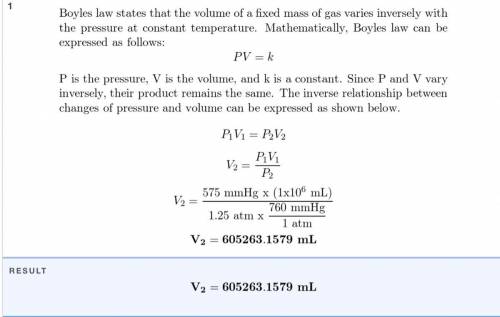
A sample of oxygen that occupies 2.9 X
10-6 mL at 635 mm Hg is subjected to a
pressure of 1.2...

Chemistry, 30.03.2021 09:00 hgdthbgjnb83661
A sample of oxygen that occupies 2.9 X
10-6 mL at 635 mm Hg is subjected to a
pressure of 1.26 atm. What will the final vol-
ume of the sample be if the temperature is
held constant?
Answer in units of mL.
help please please


Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
Questions

Mathematics, 18.10.2019 20:00


Computers and Technology, 18.10.2019 20:00

Mathematics, 18.10.2019 20:00

Geography, 18.10.2019 20:00

Mathematics, 18.10.2019 20:00

Social Studies, 18.10.2019 20:00


Mathematics, 18.10.2019 20:00


Mathematics, 18.10.2019 20:00

Mathematics, 18.10.2019 20:00

French, 18.10.2019 20:00

Mathematics, 18.10.2019 20:00

Mathematics, 18.10.2019 20:00

History, 18.10.2019 20:00

Chemistry, 18.10.2019 20:00

Computers and Technology, 18.10.2019 20:00

Biology, 18.10.2019 20:00