
Chemistry, 30.03.2021 14:00 cxttiemsp021
What is the mass of 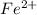 in 5 tablets of iron if the number of moles of
in 5 tablets of iron if the number of moles of 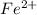 is 4.2225 x
is 4.2225 x 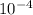 mol.
[ Ar = Fe, 56 ]
mol.
[ Ar = Fe, 56 ]

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
What is the mass of in 5 tablets of iron if the number of moles of is 4.2225 x mol.
[ Ar = Fe, 5...
Questions

Mathematics, 17.09.2019 00:30

Mathematics, 17.09.2019 00:30





Mathematics, 17.09.2019 00:30


Mathematics, 17.09.2019 00:30

English, 17.09.2019 00:30



Mathematics, 17.09.2019 00:30


Mathematics, 17.09.2019 00:30



Physics, 17.09.2019 00:30


History, 17.09.2019 00:30

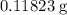 in total for the five tablets.
in total for the five tablets.  per tablet.)
per tablet.)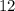 atom.
atom. 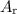 of iron is
of iron is 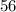 . In other words, the mass of one mole of iron
. In other words, the mass of one mole of iron 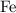 atoms would be approximately
atoms would be approximately 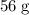 .
.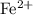 ions. Each
ions. Each 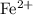 ion contains two fewer electrons than a neutral
ion contains two fewer electrons than a neutral 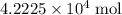 of
of 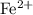 ions might be lighter than the same number of
ions might be lighter than the same number of 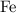 atoms by a very small extent: The mass of one mole of electrons is approximately
atoms by a very small extent: The mass of one mole of electrons is approximately 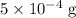 , much smaller than the mass of the same number of
, much smaller than the mass of the same number of 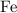 atoms (approximately
atoms (approximately 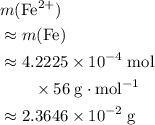 .
.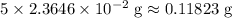 of
of 

