
Chemistry, 30.03.2021 15:50 jfitness11
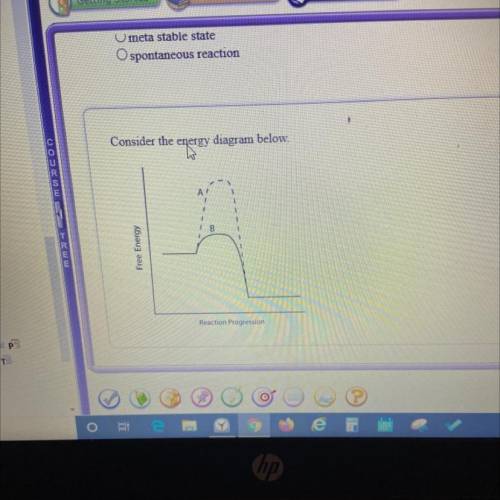
Which line indicates a higher reaction rate?
A. A because it has a lower activation energy.
B. B because it has a lower activation energy.
C. A because its Arxn is much lower.
D. B because its AGеxn is much lower.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3


Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

You know the right answer?
Which line indicates a higher reaction rate?
A. A because it has a lower activation energy.
Questions

Geography, 17.09.2021 22:20



Mathematics, 17.09.2021 22:20

Biology, 17.09.2021 22:20


Mathematics, 17.09.2021 22:20

Chemistry, 17.09.2021 22:20


Computers and Technology, 17.09.2021 22:20

English, 17.09.2021 22:20


Mathematics, 17.09.2021 22:20

History, 17.09.2021 22:20

Mathematics, 17.09.2021 22:20


Mathematics, 17.09.2021 22:20

Mathematics, 17.09.2021 22:20

Chemistry, 17.09.2021 22:20

History, 17.09.2021 22:20



