
Chemistry, 30.03.2021 22:00 Lollipop1287
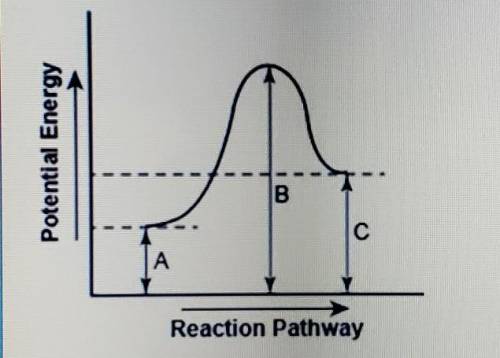
The diagram shows the potential energy changes for a reaction pathway.
part 1: describe how you can determine the total change in enthalpy and activation energy from the diagram, and if each is positive or negative.
part 2: describe how the curve will look if the reaction was exothwrmic. be sure to mention changes in the potential energies of the reactants and products and the sign changes of the enthalpy.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1
You know the right answer?
The diagram shows the potential energy changes for a reaction pathway.
part 1: describe how you can...
Questions

English, 04.03.2021 20:40

Mathematics, 04.03.2021 20:40

History, 04.03.2021 20:40



Advanced Placement (AP), 04.03.2021 20:40

Social Studies, 04.03.2021 20:40


Mathematics, 04.03.2021 20:40


Chemistry, 04.03.2021 20:40





Chemistry, 04.03.2021 20:40

Mathematics, 04.03.2021 20:40

Social Studies, 04.03.2021 20:40


English, 04.03.2021 20:40



